Method 2 Greatest Common Divisor To reduce a fraction to lowest terms (also called its simplest form), just divide both the numerator and denominator by the GCD (Greatest Common Divisor) For example, 3/4 is in lowest form, but 6/8 is not in lowest form (the GCD of 6 and 8 is 2) and 6/8 can be written as 3/42) multiply the denominator of fraction 1 by the numerator of the fraction 2 This process is called crossmultiplication Here are some examples 1/2 is equivalent to 14/28 because 1 x 28 = 2 x 14 = 28;2 Atomic Weight 2 He 400 3 Li 694 4 Be 901 5 B 1081 6 C 11 7 N 1401 8 O 1600 9 F 1900 10 Ne 18 11 Na 2299 12 Mg 2431 3B 13 Al 2698 14 Si 2809 15 P 3097 16 S 37 17 Cl 3545 18 Ar 3995 19 K 3910 Ca 4008 21 Sc 4496 22 Ti 4787 23
1
28 14 si 4 2 he
28 14 si 4 2 he-In chemistry and physics, the iron group refers to elements that are in some way related to ironThese elements are relatively abundant both on Earth and elsewhere in the universe The term is ambiguous in different contexts, and almost obsolete in chemistry2 He 1s 2 Helium 0 14 Si Ne3s 2 3p 2 Silicon 4,2 15 P Ne3s 2 3p 3 Phosph orus ±3,5,4 16 S Ne3s 2 3p 4 Sulfur 365 ±2,4,6 17 Cl Ne3s 2 3p 5 Chlorine ±1,3,5,7 18 Ar Ne3s 2 3p 6 Argon 32 Ge Ar3d 10 4s 2 4p 2 Germanium




Practice Drawing Atoms Drawing Atoms Rules Protons Atomic Number Electrons Atomic Number Neutrons Mass Number Atomic Number 1 St Level Can Hold Ppt Download
Stepbystep explanation 2 1 4 (∵ 14 used to simply) grendeldekt and 28 more users found this answer helpful heart outlined28 Si 14 31 P 15 32 S 16 355 Cl 17 40 Ar 18 59 Ni 28 635 Cu 29 65 Zn 30 70 Ga 31 73 Ge 32 75 As 33 79 Se 34 80 Br 35 84 Kr 36 106 Pd 46 2 He Helium 32 Ge Germanium 3 Li Lithium 33 As Arsenic 4 Be Beryllium 34 Se Selenium 514Si does an alpha decay?
Isaiah 2814 It is bad with a people when their thrones of judgment become the seats of the scornful, when rulers are scorners;Identify the emission particle from potassium40 alpha particle betaplus particle electron capture betaminus particle A The isotope ²⁰⁶Po has a halflife of days If you have 364 x 10^27 atoms of this isotope, how long will it take for the sample to Si Silicon 2 8 5 15 P Phosphorus 2 8 6 16 S Sulfur 36 2 8 7 17 Cl Chlorine 3545 2 8 8 18 Ar 2 8 18 32 14 2 76 Os Osmium 2 8 18 32 15 2 77 Ir Iridium 2 8 18 32 17 1 78 Pt Platinum 2 8 18 32 18 1 79 Au 2 8 18 28 8 2 66 Dy Dysprosium 2 8 18 29 8 2 67 Ho Holmium 164
Li 3 4 3 P 15 16 15 Cl 17 18 17 Ni 59 28 28 K 19 19 Ag 108 47 47 H 1 0 1 Si 14 28 14 W 17 174 17 Ne 10 10 NOTE The number protons and electrons is equal to the atomic number To find neutrons, subtract the number of protons from the atomic mass To find the atomic mass, add the number of protons and neutrons O Oxygen226Ra → 4 2 He 222 86 Rn (b) 84 35 Br → 4 2 He 80 33 As (c) 242 94 Pu → 4 2 He 240 92 U (d) 14 7 N 41 1 p → 2 He 11 6 C (e) 18 8 O 01 0 n →1 e 19 9 F 4 (a) 235 92 U 1 0 n → 147 59 Pr 86 33 As 3 0 n (b) This is a nuclear fission reaction 5 (a) 31 15 P 1 1 p → 28 14 Si 4 2 He artificial transmutation (b) 27 13 Al 4 2 He4 He 2 11 B 5 12 C 6 14 N 7 16 O 8 19 F 9 Ne 10 27 Al 13 28 Si 14 31 P 15 32 S 16 355 Cl 17 40 Ar 18 59 Ni 28 635 Cu 29 65 Zn 30 70 Ga 31 73 Ge 32 75 As 33 79 Se 34 80 Br 35 84 Kr 36 106 Pd 46 108 Ag 47 112 Cd 48 115 In 49



2
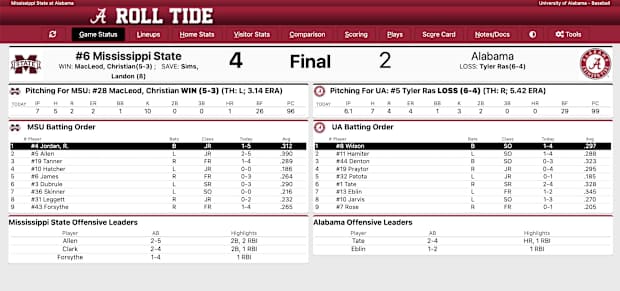



Alabama Baseball Falters Late Against No 6 Mississippi State 4 2 Sports Illustrated Alabama Crimson Tide News Analysis And More
Click here👆to get an answer to your question ️ Calculate Q value of the following nuclear reaction 13^Al^27 2^He^4 14^Si^30 1^H^1 Q The exact mass of 13^Al^27 is amu , 14^Si^30 is amu , 2^He^4 is amu and 1^H^1 is amuHe addressed himself to the scornful men who ruled in Jerusalem, who were the magistrates of the city, Isaiah 2814;Therefore hear the word of the LORD, you scoffers who rule this people in Jerusalem You boast, "We have entered into a covenant with death, with the realm of the dead we have made an agreement When an overwhelming scourge sweeps by, it cannot touch us, for we have made a lie our refuge and falsehood our hiding place" So this is what the Sovereign LORD says "See, I lay




Hydrogen Bridged Oligosilanylsilyl Mono And Oligosilanylsilyl Dications Nimoth Chemistry A European Journal Wiley Online Library
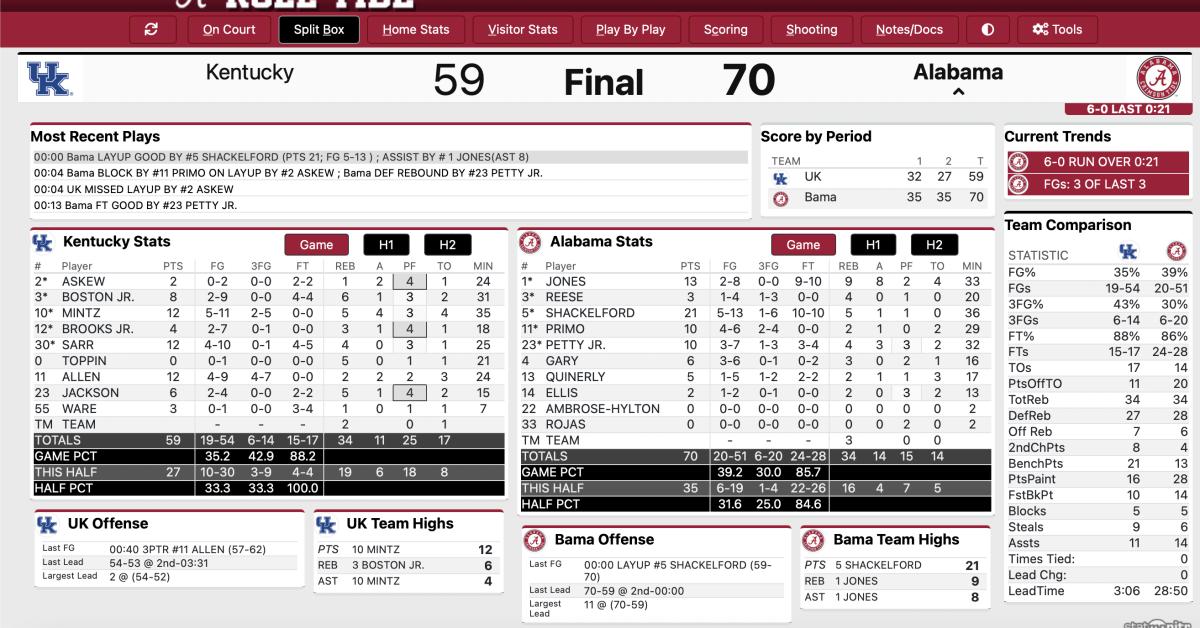



Live Updates Kentucky Wildcats At No 9 Alabama Crimson Tide Basketball Sports Illustrated Alabama Crimson Tide News Analysis And More
2 2 He 4 2752(−1) BB, H(CNO, pp) 3 8 O 16 9592(−3) He 4 6 C 12 3032(−3) He 5 10 Ne 1548(−3) C 6 26 Fe 56 1169(−3) NSE 7 7 N 14 1105(−3) H(CNO) 8 14 Si 28 6530(−4) O 9 12 Mg 24 5130(−4) C, Ne 10 16 S 32 3958(−4) O 11 10 Ne 22 76(−4) He 12 12 Mg 26 72(−5) C, Ne 13 18 Ar 36 7740(−5) Si, O 14 26 Fe 54 7(28/14) si^4 this atom contains 14 protons?At the end of their lives, once other fuels have been exhausted, such stars can enter a brief phase of " silicon burning " This involves the sequential addition of helium nuclei 4 2 He (an " alpha process ") to the heavier elements present in the star, starting from 28 14 Si



Frontiers The Role Of Electrical Impedance Tomography For Management Of High Risk Pulmonary Embolism In A Postoperative Patient Medicine



Materials Free Full Text Effect Of Silica Fume On Metakaolin Geopolymers Sulfuric Acid Resistance Html
2 He 40 3 Li 69 4 Be 90 5 B 108 6 C 1 7 N 140 8 O 160 9 F 170 10 Ne 2 11 Na 230 12 Mg 243 13 Al 270 14 Si 281 15 P 310 16 S 321 17 Cl 355 18 Ar 400 19 K 391 Ca 401 21 Sc 450 22 Ti 479 23 V 509 24 Cr 5 25 Mn 549 26 Fe 558 27 Co 5 28 Ni 587 29 Cu 635 30 Zn 654 31 Ga 697 32 Ge 726 33 As 749 34 Se 790 35This preview shows page 696 702 out of 800 pagesLi 3 4 3 P 15 16 15 Cl 17 18 17 Ni 59 28 28 K 19 19 Ag 108 47 47 H 1 0 1 Si 14 28 14 W 17 174 17 Ne 10 10 NOTE The number protons and electrons is equal to the atomic number To find neutrons, subtract the number of protons from the atomic mass To find the atomic mass, add the number of protons and neutrons O Oxygen




Solved Calculate The Total Binding Energy For 28 14si The Chegg Com
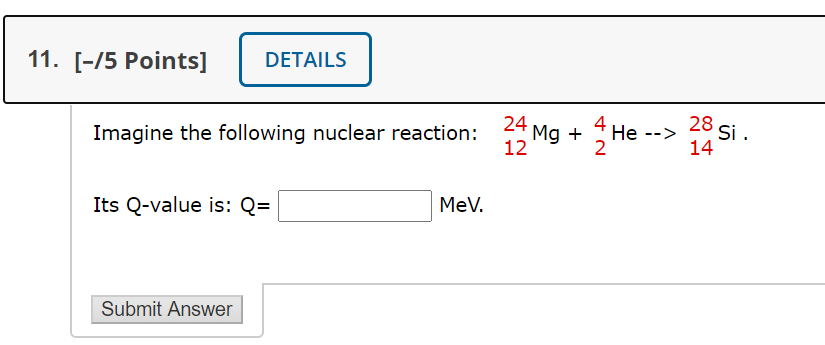



Solved 11 75 Points Details Imagine The Following Chegg Com
1 IA Noble Gas 1 H 1 1 2 IIA 13 IIIA 14 IVA 15 VA 16 VIA 17 VII 2 Oxidation States He ‐ The most common oxidation state(s) is shown in red A 3 Li 1 4 Be 2 5 B 3 6 C 4 2 ‐4 7 N 5 4 3 8 O ‐1 ‐2 9 F ‐1 10 Ne The most common oxidation state(s) is shown in redAnswers 2 Get Other questions on the subject Chemistry Chemistry, 0030, tdowling331 What must happen before a body cell can begin mitotic cell division Answers 2 continue Chemistry, 0400, NotCJ9006 Propane is a gas in its natural state why and how is propane sold asMostly in period (row) 4 of the periodic table The term has different meanings in different contexts In chemistry, the term is largely obsolete, but it often means iron, cobalt, and nickel, also called the iron triad;



Tables Of Piping Standards Soft Copper G Asket Xx Stronc T It E D H O Les L F Q H M H T Gt Size I R Gt O D Wo Size B C 3 4 42 I Os
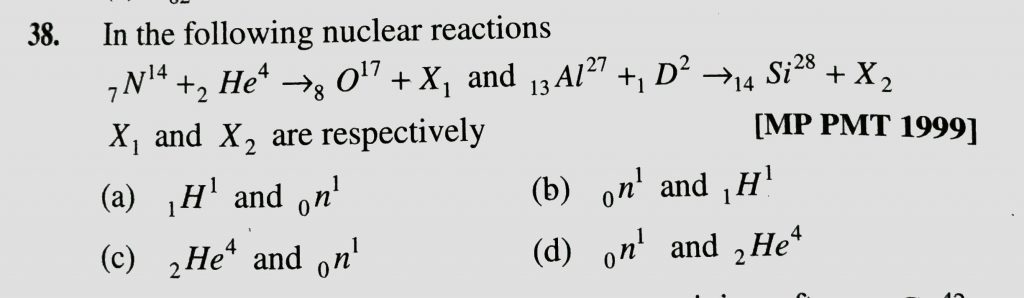



In The Following Nuclear Reactions 7n 14 2he 4 To 8o 17 1x And 13al 27 1d 2 To 14si 28 X2 Xi And X2 Are Respectively Sahay Sir
Mostly in period (row) 4 of the periodic table The term has different meanings in different contexts In chemistry, the term is largely obsolete, but it often means iron, cobalt, and nickel, also called the iron triad;28 14 Si 4 2 He 9593 MeV (34%) → 31 15 P 1 1 H 7676 MeV (56%) → 31 16 S 27 13 al 1 0 n 28 14 si a 4 2 he b 0 1 e c 1 0 n d 0 School George Washington University;




Solved Complete The Following Nuclear Equation 238 U 298 Chegg Com




Shell Model For Proton And Neutron Showing How Triton Rich Nuclei 24 8 Download Scientific Diagram
11 (a) A nucleon is any particle contained in the nucleus of the atom, so it can refer to protons and neutrons (b) An α particle is one product of natural radioactivity and is the nucleus of a helium atom (c) A β particle is a product of natural radioactivity and is a highspeed electron (d) A positron is a particle with the same mass asIn chemistry and physics, the iron group refers to elements that are in some way related to iron;The subscripts and superscripts are necessary for balancing nuclear equations, but are usually optional in other circumstances For example, an alpha particle is a helium nucleus (He) with a charge of 2 and a mass number of 4, so it is symbolized latex{}_{2}^{4}\text{He}/latex



Florida State Sweeps Miami 3 Game Changing Plays Sports Illustrated Florida State Seminoles News Analysis And More



Journal And Proceedings Of The Royal Society Of New South Wales Secccoti Cti Toltqh 7t6u 7 N T 34r Ne Saerritt E Position A Tnzj Ct Sects Of Coccnzyty Lt Al Society N S W 17 Plate 23 Pan Iii To Milsons
The mass of 27 13Al is u, the mass of 42He is u, the mass of 30 15P is 2997 u, and the mass of n is uView Homework Help Chapter 43 from PHY 11 and 21 at Lehigh University 431 a) b) c) 85 37 5 81 28 14 Si has 14 protons and 14 neutrons Rb TlMethod 2 To reduce a fraction to lowest terms (also called its simplest form), just divide both the numerator and denominator by the Greatest Common Factor (GCF or GCD) For example, 2/3 is in lowest form, but 4/6 is not in lowest form (the GCD of 4 and 6



Live Updates No 4 Alabama Vs Tennessee Sports Illustrated Alabama Crimson Tide News Analysis And More




Dxdi 553nkq 3m
14 Si silicon 281 15 P phosphorus 310 16 S sulfur 321 17 Cl chlorine 355 18 Ar argon 399 19 K potassium 391 Ca calcium 401 21 Sc scandium 450 22 Ti titanium 479 23 V vanadium 509 24 Cr chromium 5 25 Mn manganese 549 26 Fe iron 558 27 Co cobalt 5 28 Ni nickel 587 29 Cu copper 635 30 Zn zinc 654 31 Ga28 14 Si Question 28 14 Si Express your answers as integers separated by commas Could you please explai how to do t28 14SiÆ 4 2He 24 12Mg 2 14 24 3 21 12 4 28 6 5 35 3 25) If you have 50 grams of an isotope of Plutonium239, that has been in a sealed container for 2100 years and the halflife is 300 years, how much Plutonium239 did you originally start with?




Experimental Data For The 28 Si 30 Kev 4 10 18 Cm 2 And 31 P Download Scientific Diagram




Diluted Acid Blank On The 28 Si And 30 Si Isotopes Where 14 N 2 And 14 Download Scientific Diagram
2/4 is equivalent to 14/28 because 2 x 28 = 4 x 14 = 56;Anytime you Catholic, this would get a value of minus 2995 times 10 to the minus three atomic mass movements but this minus signs Very important because it tells us the energy was absorbed in his reaction So now we can actually you're released something like that on so square inputting numbers Consider the nuclear reaction $^{28}_{14}SiRadiation Science and Engineering Center The Pennsylvania State University University Park, PA 2 17 92 U 235 0 n 1 → 42 Mo 95 _____ 2



1




In The Following Reaction 12 Mg 24 2 He 4 To 14 Si X 0 N 1 Xis A 28 B 27 C 26 D 22 Snapsolve
3/6 is equivalent to 14/28 because 3 x 28 = 6 x 14 = 84Categories Chemistry Leave a Reply Cancel reply Your email address will not be published Required fields are marked * Comment Name *Transcribed image text The missing product from this reaction is _____^27_13 Al ^1_0 n rightarrow^28_14 Si _____ A)^4_2 He B)^0_1 e C)^1_0 n D)^0_1 e E)^0_0 gamma The missing product from this reaction is _____^10_5 B _____ rightarrow^13_7 N ^1_0 n A)^4_2 He B)^0_1 e C)^1_0 n D)^0_1 e E)^0_0 gamma The missing product from this reaction is _____^221_87 Fr




Practice Drawing Atoms Drawing Atoms Rules Protons Atomic Number Electrons Atomic Number Neutrons Mass Number Atomic Number 1 St Level Can Hold Ppt Download




Ch 4 Atomic Structure History Ppt Download
230 15P correct 327 14Si 426 12Mg 531 15P 628 14Si Explanation 27 13Al 4 2He −→ n 3015P 002(part2of2)100points What was the Q value of reaction?4 1 1 H → 4 2 He 1 e 0 0 γ 0 0 ν (267 MeV) 28 14 Si, 32 16 S → 56 26 Fe, 56 28 Ni 1 s explosive fusion → light elements neutron capture → heavy elements How to Cook Everything Mix it all up and get everything from hydrogen to uranium (and maybe even up to californium)Periodic Table The periodic table of elements is an arrangement of the chemical elements ordered by atomic number in columns (groups) and rows (periods) presented so as to show their periodic nature Point at or click an element from the periodic table for more information ***Groups are by 3 notation conventions
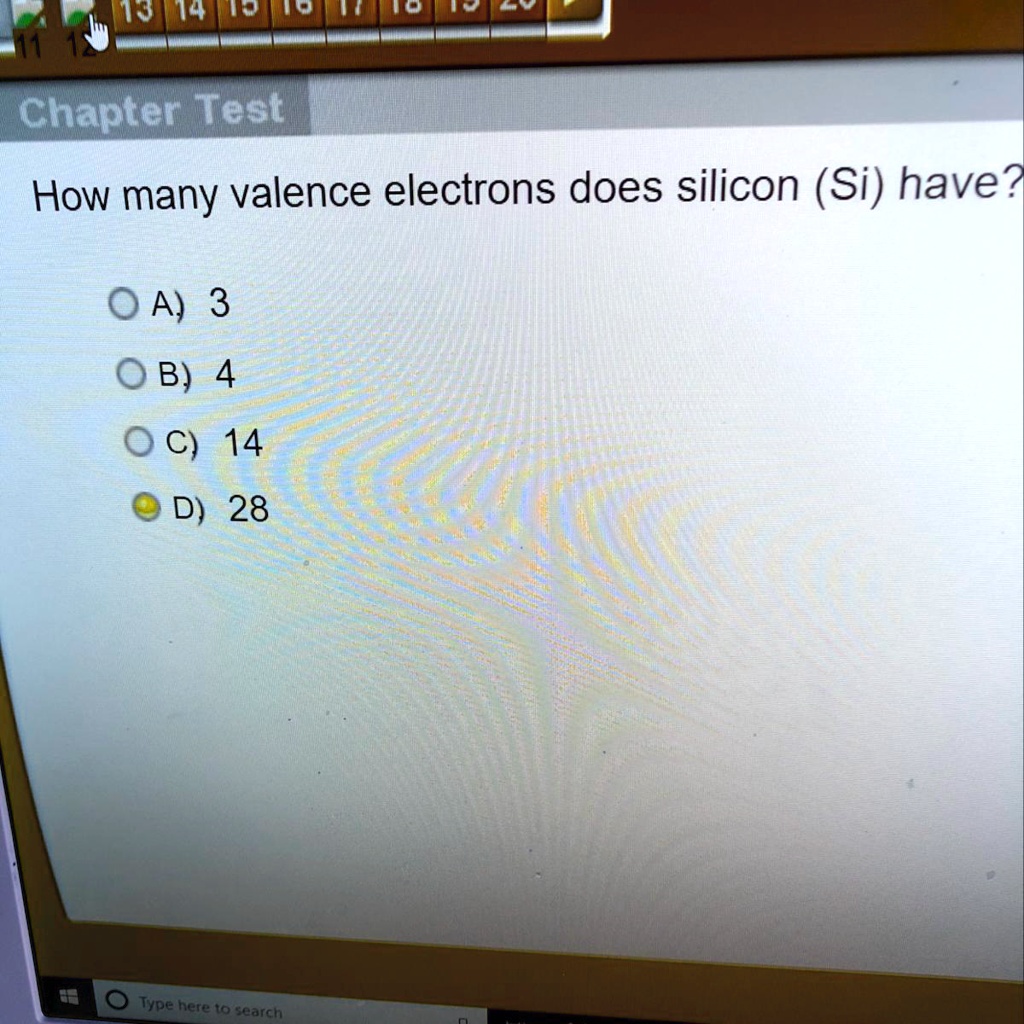



Solved How Many Valence Electrons Does Silicon Si Have 19 44 Ht Hd 2 Hi Len Chapter Test How Many Valence Electrons Does Silicon Si Have 0 A 3 0 B 4 0 C 14 D 28 Type Here T0 Search




29 Si Mas Nmr Spectra Of B1 Samples Calcined In Ammonia A And In Download Scientific Diagram
In chemistry and physics, the iron group refers to elements that are in some way related to iron;1 day ago I Tried To Wear The World 2 10 Live At Mark Lamarr Show, Radio1, Maida Vale Studios, London, UK 1112 Live At Creation Records Birthday Party, Royal Albert Hall, London, UK 13 Unknown Date and Venue Live Gig 1417 Live At Shepherd's Bush Empire, London, UK 18 Unknown Date and Venue Live Gig 19 Live At Later With JoolsOr, sometimes, other elements that resemble iron in some chemical aspects




Comparison Of The Real Potentials Of The 8 B 28 Si Interaction Download Scientific Diagram
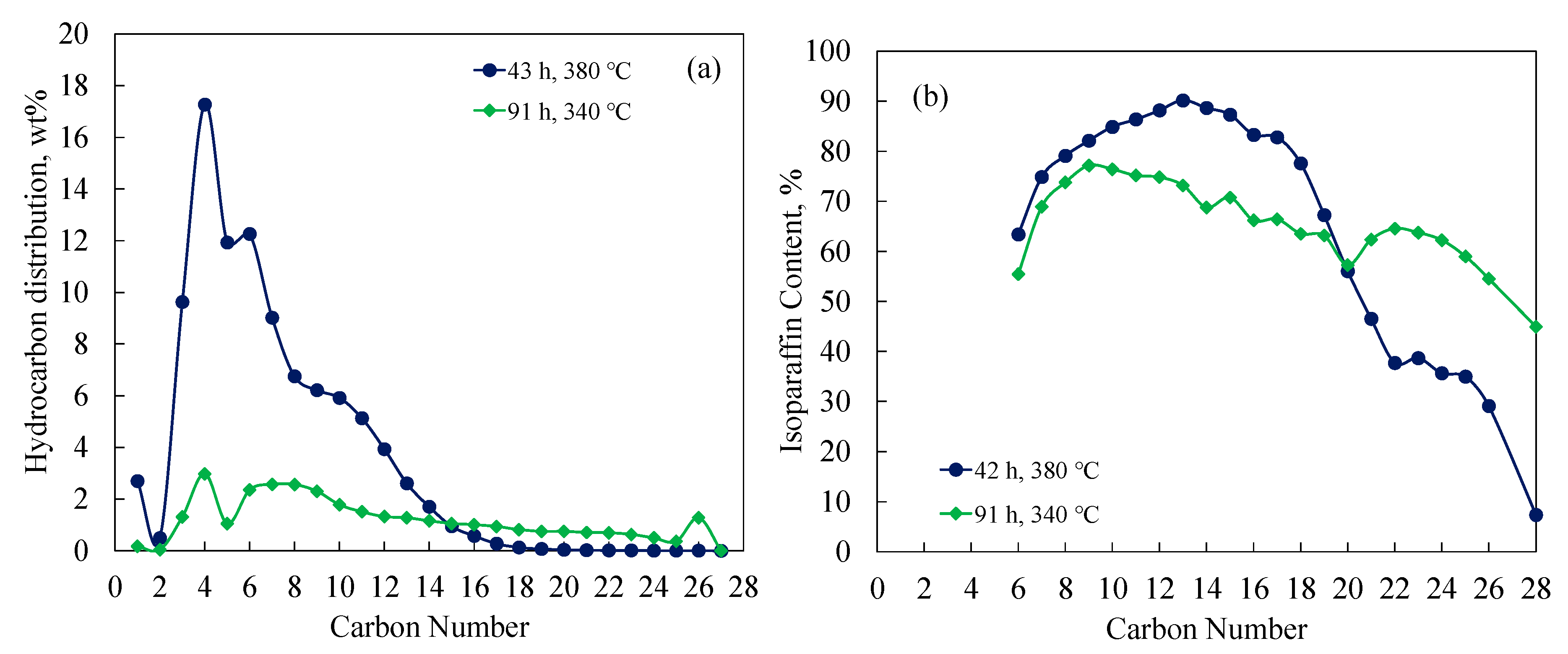



Reactions Free Full Text Hydrocracking Of Octacosane And Cobalt Fischer Tropsch Wax Over Nonsulfided Nimo And Pt Based Catalysts Html
13 Aluminium (Al) 27 13 13 13 14 2 8 3 14 Silicon (Si) 28 14 14 14 14 2 8 4 15 Phosphorus (P) 31 15 15 15 16 2 8 5 16 Sulphur (S) 32 16 16 16 16 2 8 6 17 Chlorine (Cl) 35 17 17 17 18 2 8 7 18 Argon (Ar) 40 18 18 18 22 2 8 8 19 Potassium (K) 39 19 19 19 2 8 8 1But that the rulers of Jerusalem should be men of such a character, that they should make light of God's judgments and scorn to take notice of the«Горіння» кисню — утворення 28 14 Si, 32 16 S «Горіння» неону — утворення 36 18 Ar, 40 Ca «Горіння» кремнію — утворення 52 26 Fe, 56 28 Ni;




28 2 Efficient Outdoor Stable Perovskite Silicon Tandem Solar Cell Sciencedirect




Pga Tour Communications Groups For The 22 World Golf Championships Dell Technologies Match Play T Co Nbajjatjnh Twitter
Or, sometimes, other elements that resemble iron in some chemical aspectsThen, we divide both 28 and 14 by the greatest common factor to get the following simplified fraction 2/1 Therefore, this equation is true 28/14 = 2/1 If the numerator is greater than or equal to the denominator of a fraction, then it is called an improper fraction In that case, you could convert it into a whole number or mixed numberAnswers 2 Show answers Another question on Chemistry Chemistry, 0350 Afission reaction that occurs when uranium 235 absorbs a neutron leads to the formation of barium141 and krypton92
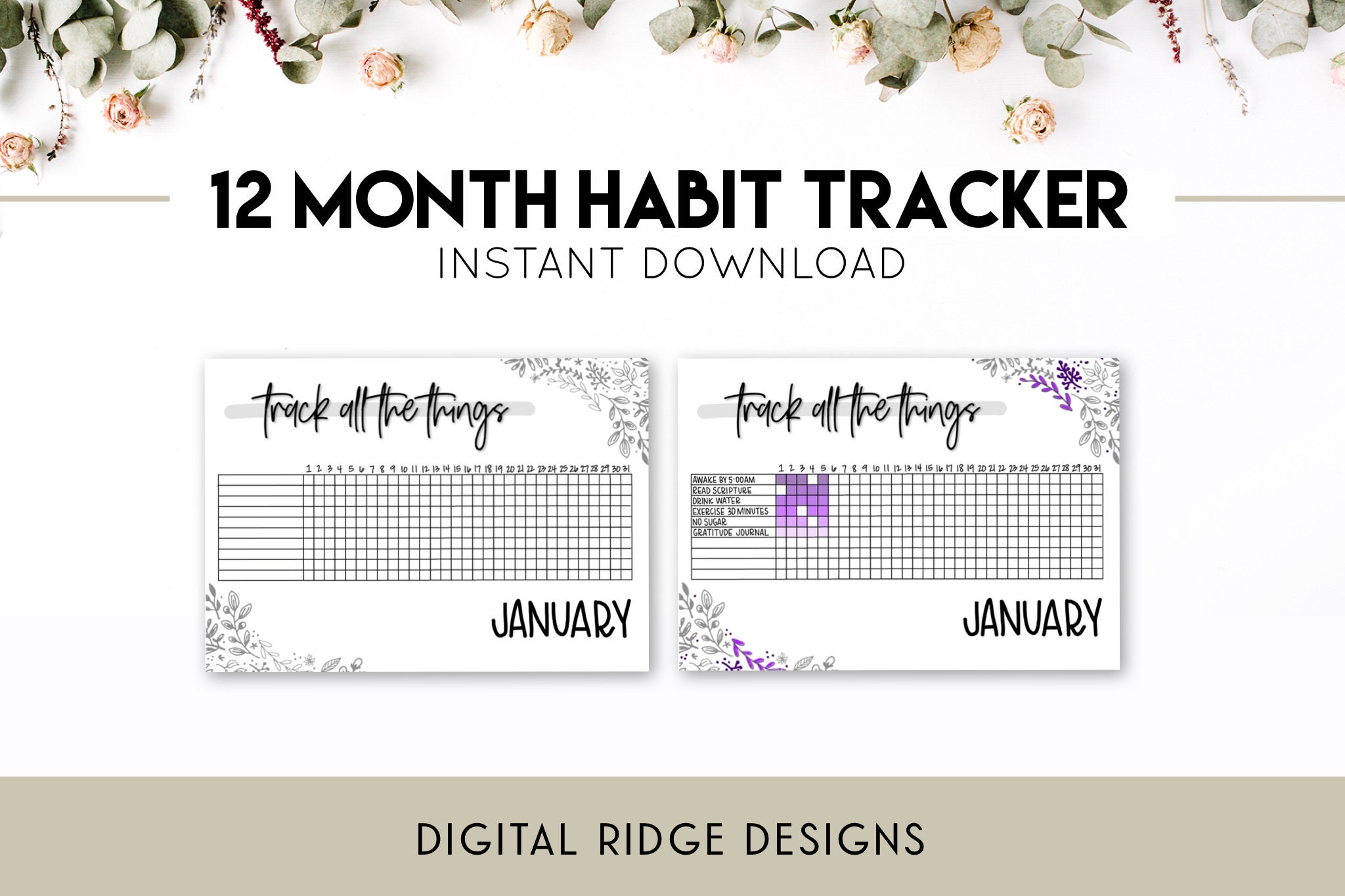



12 Month Habit Tracker Instant Download Digital Planner Etsy Ireland




1 Gcse Questions And Answers Calculations Remember That You Can Search Using Edit 6 Consecutive Gcse Chemistry Papers Ppt Download
Answer choices 14 7N 4 2 He 1 1 H 17 8 O s Question 32 SURVEY 30 seconds Q Brain tumors can be located by using an isotope ofNuclear fusion sequence and silicon photodisintegration After a star completes the oxygenburning process, its core is composed primarily of silicon and sulfur If it has sufficiently high mass, it further contracts until its core reaches temperatures in the range of 27–35 GK (230–300 keV)At these temperatures, silicon and other elements can photodisintegrate, emitting a proton or30 14 Si 30 15 P 28 12 Mg 28 13 Al s Question 24 SURVEY 30 seconds Q During a fission reaction, which type of particle is captured by a nucleus?




Discovery And Evolution Of 12n Substituted Aloperine Derivatives As Anti Sars Cov 2 Agents Through Targeting Late Entry Stage Sciencedirect




If The Atomic Weight Of C And Si Are 12 And 28 Respectively Then What Is The Ratio Of The Youtube
Using Fig 29 from our textbook (p 48, Lilley), justify the nuclear spin and parity ( for even, and − for odd) for the following nuclei 3 2 He(1 2 )4 2 He(0) 27 13 Al(5 2 28 14 Si(0) 38 18 Ar(0) 41 19 K(3 2 ) 63 29 Cu(3 2 −) 65 29 Cu(3 2 −) 64 30 Zn(0) Question 2 What is the minimum photon energy required to dissociate 2HConsider the three equations below 14/7N1/1H—>15/8O 4/2He4/2He—>8/4Be 28/14Si7 4/2He—>56/28Ni which statement do these reactions best support?Рівноважний процес (eпроцес) Утворення елементів, важчих від заліза



The American Anti Slavery Almanac For Calculated For Boston New York And Pittsburgh 15 4 36 6 24 11 14 5 3 8 14 Tuesd P 6 7 13 481 18 4 25




File Tables Of Piping Standards 1919 Jpg Wikimedia Commons
Course Title CHEM 1111;ATOMIC WEIGHTS OF THE ELEMENTS 19 These tables are based on the 15 table with changes from the 15 table for the values of aluminium, argon, cobalt, gold, holmium, iridium, manganese, niobium, praseodymium, protactinium, rhodium, terbium, thulium and yttrium See report 5 June 18The revised value of hafnium was reported 11 December 19Type Test Prep Uploaded By UltraHawkMaster16 Pages 800 Ratings % (141) 124 out of 141 people found this document helpful;




File Periodic Table Meyer 1864 Png Wikipedia




Characterization Of Chemical Accelerators For Sustainable Recycling Of Fresh Electric Arc Furnace Dust In Cement Pastes Sciencedirect
(28/14) si^4 this atom contains 14 protons?
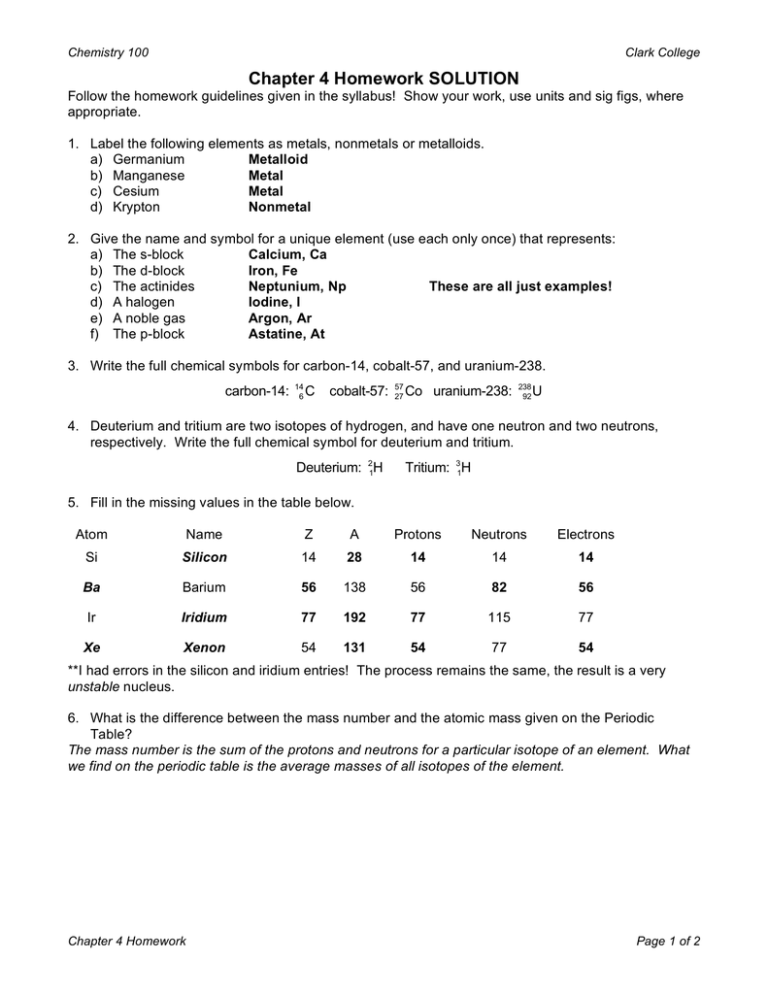



Chapter 4 Homework Solution




Ekaboron 44 And Its Analogies See Fig 2 Download Scientific Diagram



Global Bio Silicon Maps




Periodic Table Summary Of Anything Docsity



Application Of Different Options Of Combined Inhalation Anesthesia For Intraocular Interventions In Children
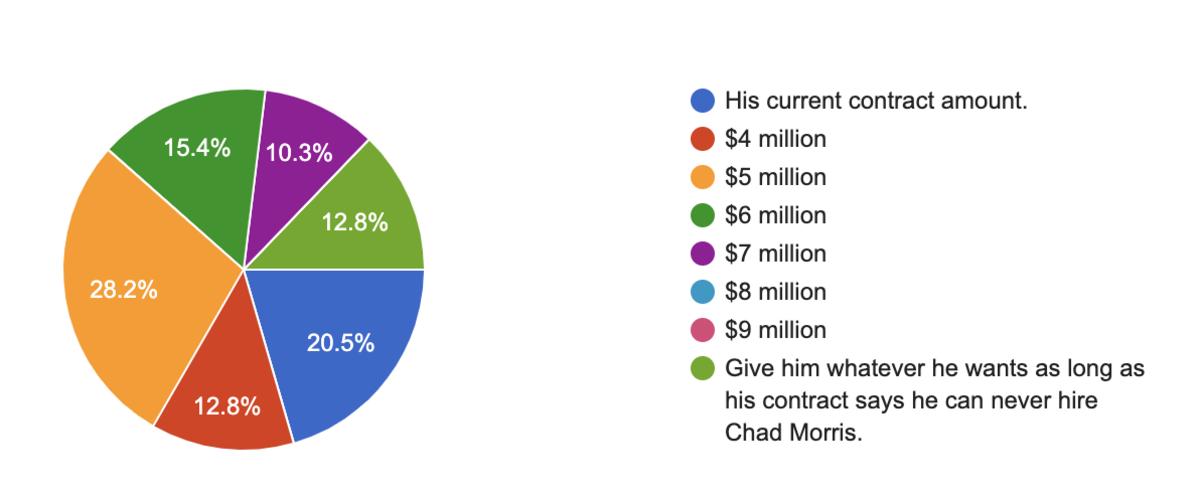



Razorback Fans Were Asked Whether They Would Meet Jimmy Sexton S Demands To Double Sam Pittman S Salary All Hogs
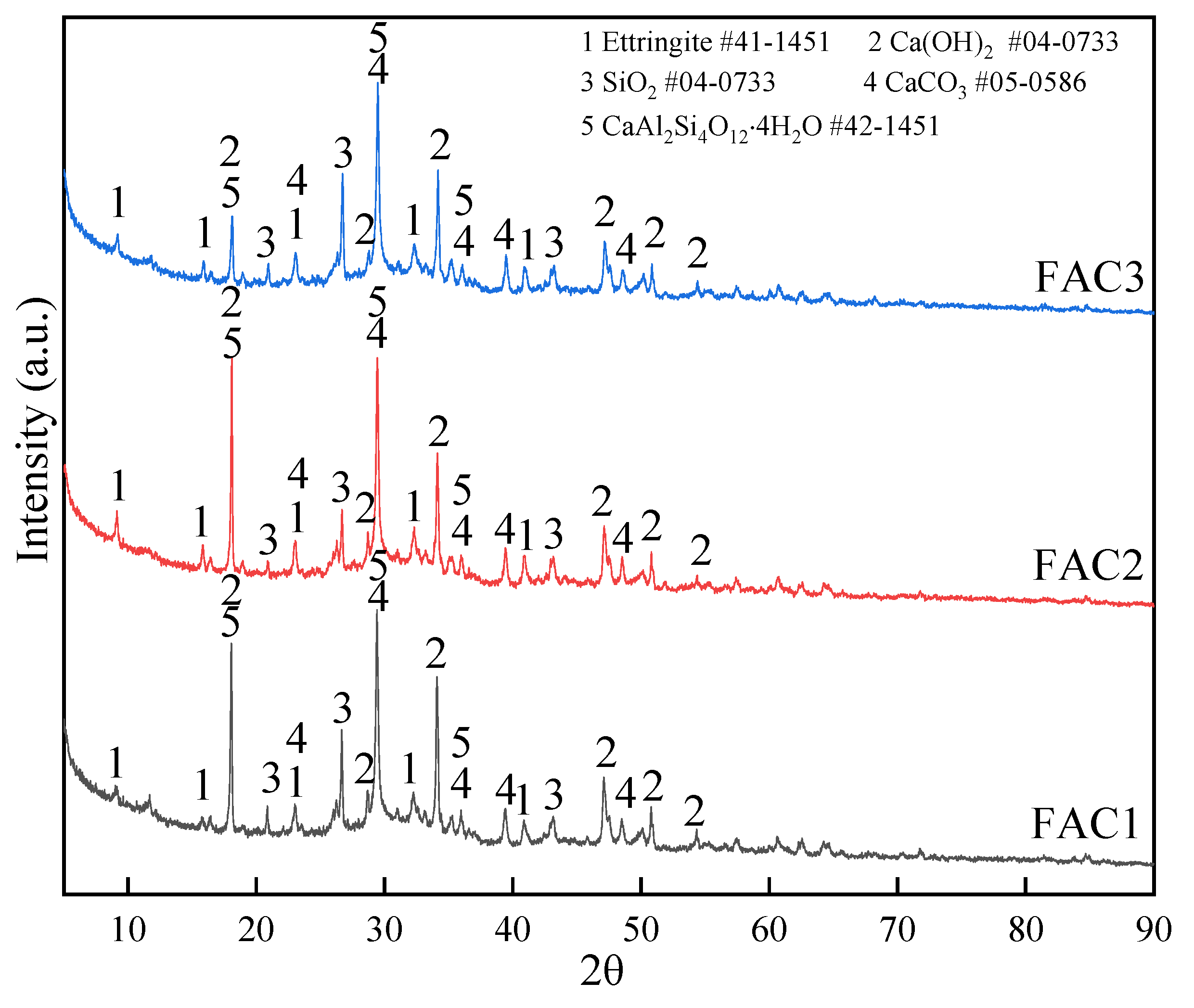



Materials Free Full Text Effect Of Electrolytic Manganese Residue In Fly Ash Based Cementitious Material Hydration Behavior And Microstructure Html
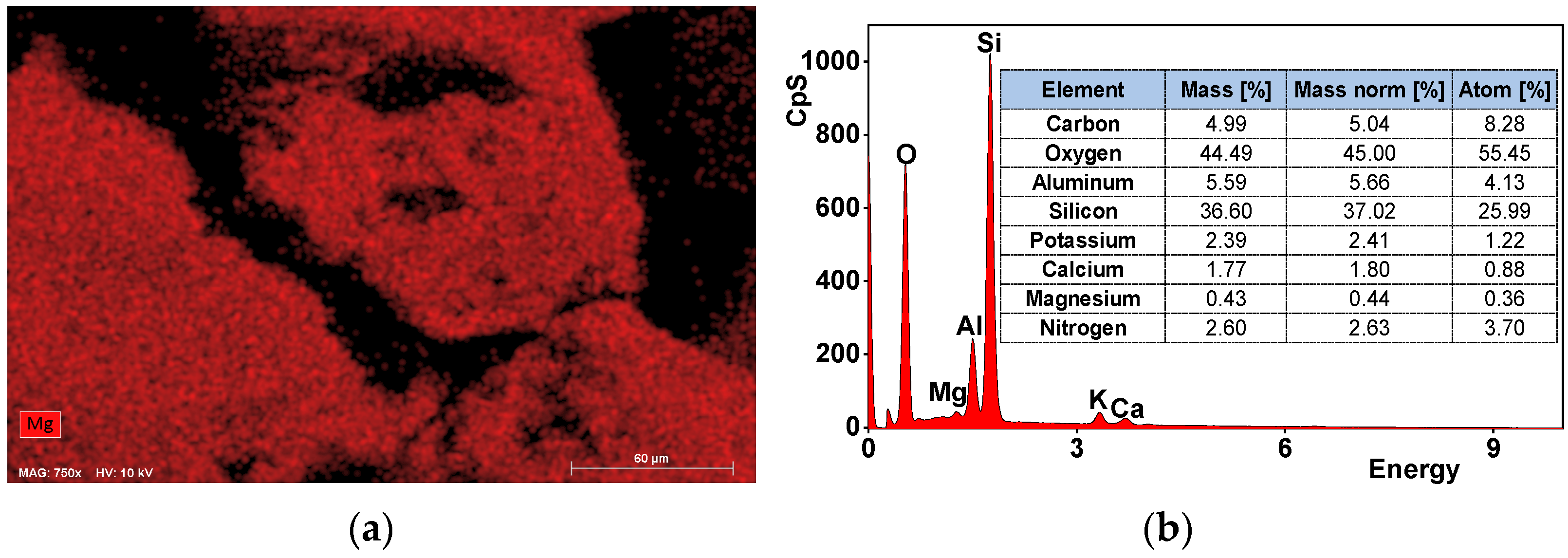



Materials Free Full Text Nutrients Recovery From Dairy Wastewater By Struvite Precipitation Combined With Ammonium Sorption On Clinoptilolite Html



Bioscience West 22 Silicon Maps




Schematic Of The Sif 4 And 28 Sih 4 Distillation System 1 Download Scientific Diagram




Ufc Vegas 29 The Korean Zombie Vs Ige Mma Betting And Dfs Breakdown Si Fantasy Pro On Sports Illustrated Vegas Best Bets Inside Info Dfs Analysis Tools More




Monoclonal Antibody Mediated Neutralization Of Sars Cov 2 In An Irf9 Deficient Child Pnas




Principles Of Food Science 4th Edition Page Iii 3 Of 0




Solved Balance The Following Reaction 25 Mg 12 4 He 2 H Chegg Com



Silicon Atomic Number




The 28 Si 180 Hf And The Ratio Signals Of The Hfo 2 Layer Download Scientific Diagram




Are The Atomic Nuclei Spatial Grids Of Particles




Periodic Table Atomic Mass Trick To Learn Atomic Mass Number Youtube



1



Studypool Com
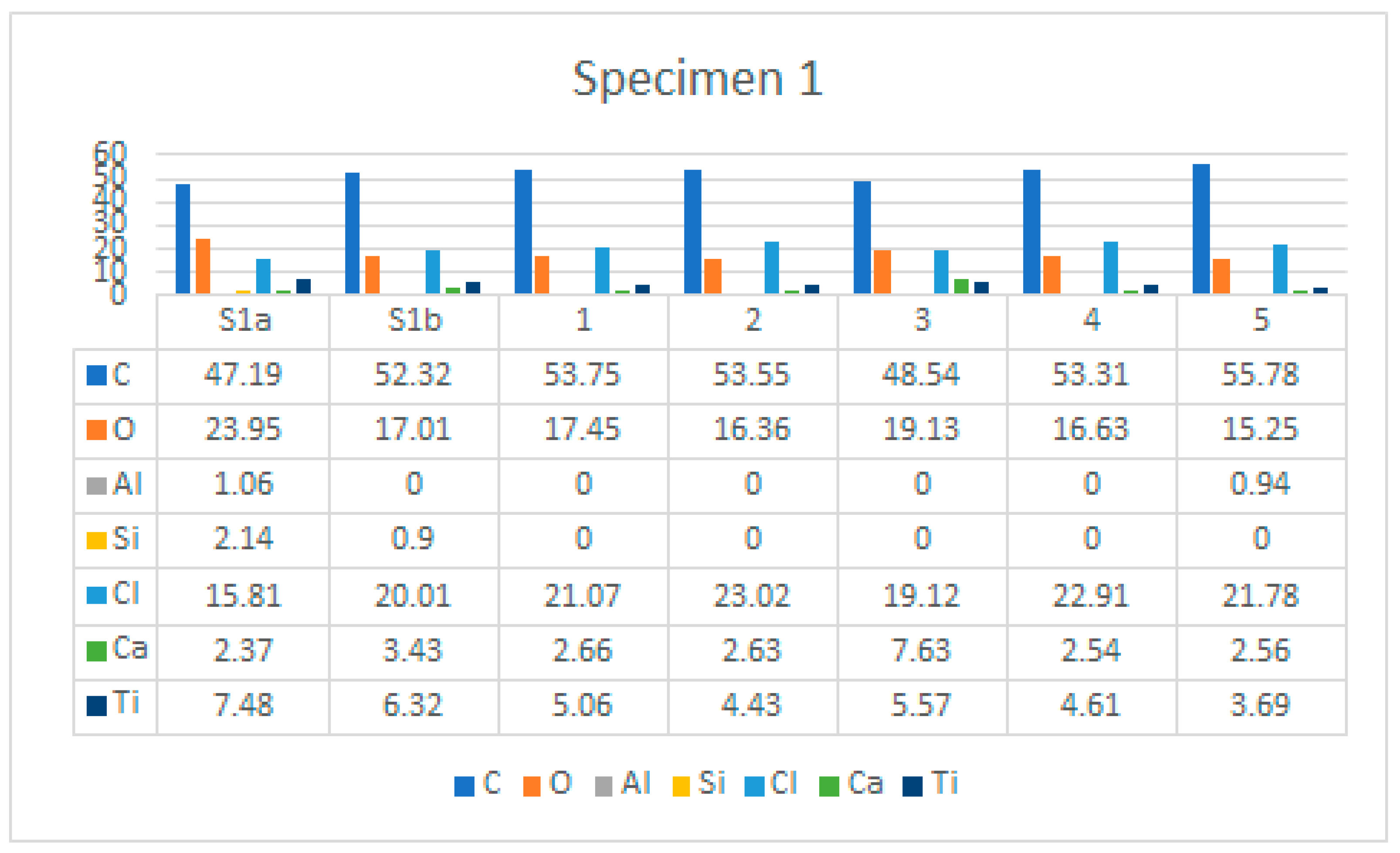



J8saj Etufnojm




Chapter 1 Structure And Bonding Ppt Download



Sessional Papers 8 To 9 12 6 17 8 18 8 19 8 3271 53 4 46 39 32 10 30 10 46 12 12 28 12 21 2 8 238 58 24 D I I U O To A 1215i2i6 12 15 1216 1216 1218 Min 19 19 16 5 O Ia Sl D Ill Si M 59 850 841 8 31 8 24 1 13 815 69 I C 6
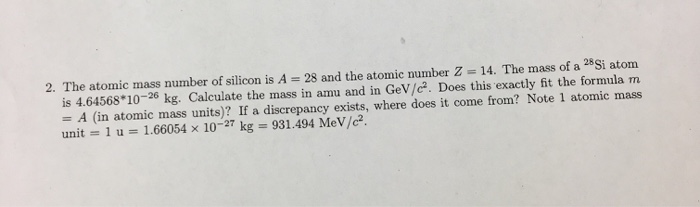



Solved The Atomic Mass Number Of Silicon Is A 28 And The Chegg Com




Pdf Inference Of The Local Interstellar Spectra Of Cosmic Ray Nuclei Z 28 With The Galprop Helmod Framework Semantic Scholar



Question C9ba9 Socratic




Silicon Key Stage Wiki
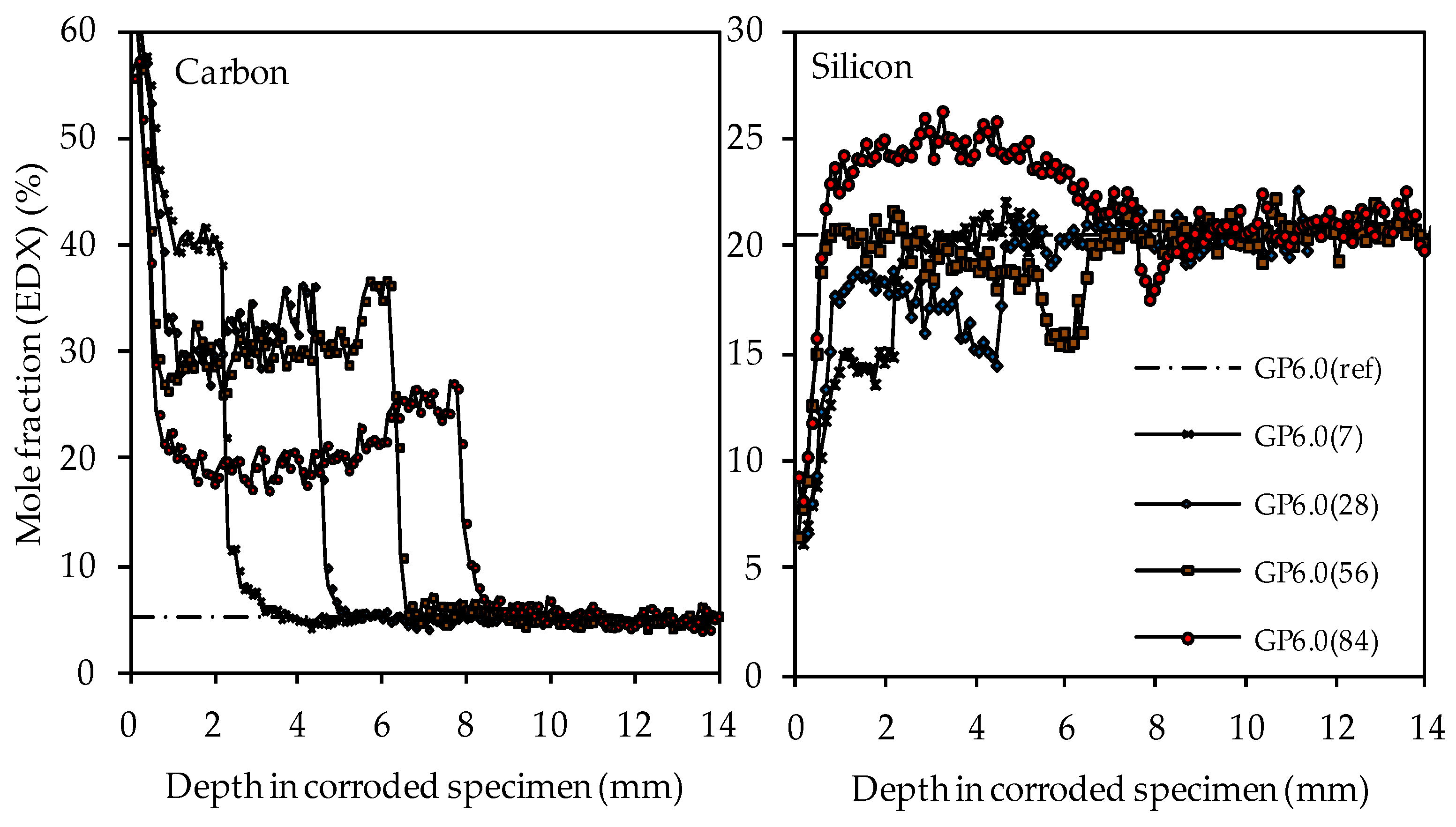



Materials Free Full Text Effect Of Silica Fume On Metakaolin Geopolymers Sulfuric Acid Resistance Html




Solved 14 Using The Periodic Table Write The Name And Symbol Of The Element With The Following Atomic Number 2 28 B 2 13 Z 4 2 33 2 50 15 Complete The Following Table For
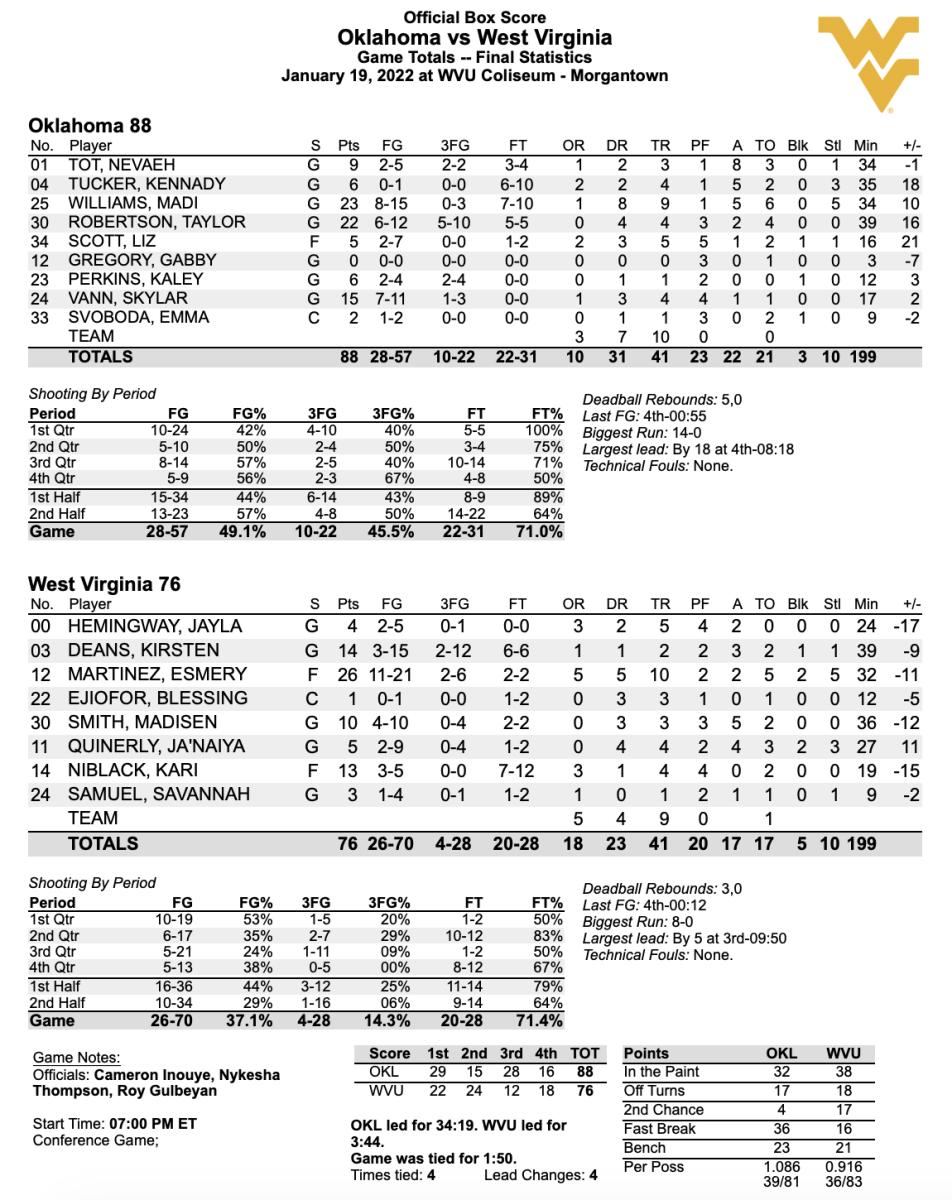



Oklahoma Pulls Away From Wvu To Continue Road Dominance Sports Illustrated Oklahoma Sooners News Analysis And More
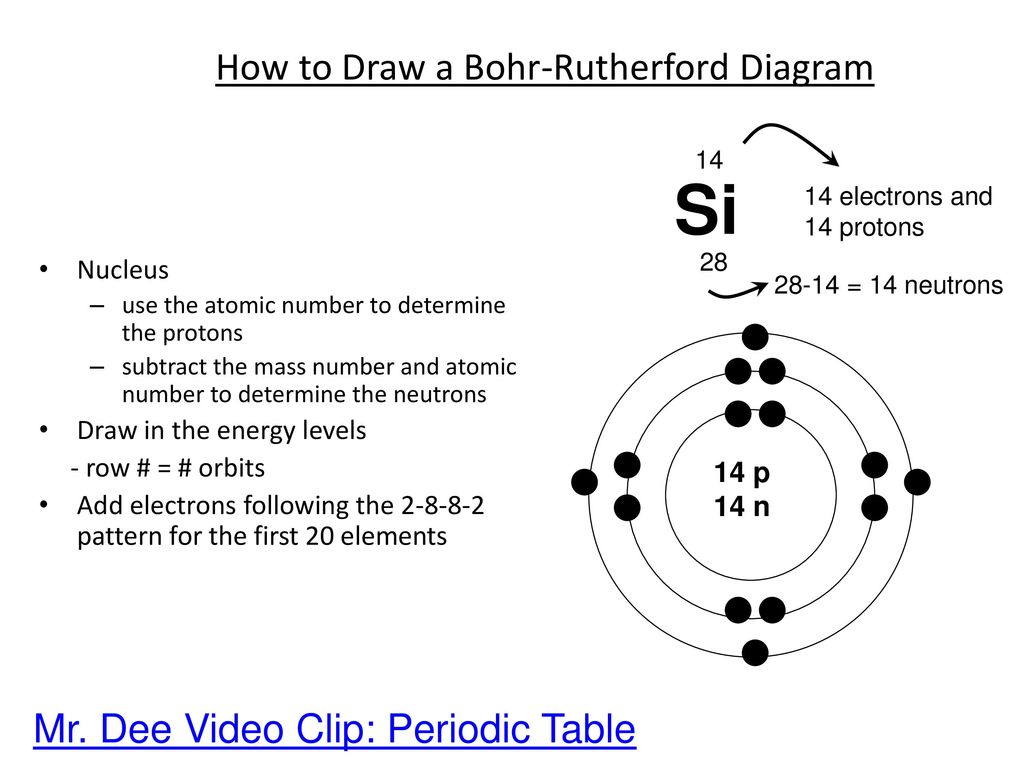



The Structure Of The Atom Ppt Download




Christopher G Hamaker Illinois State University Normal Il Ppt Video Online Download




Solved Example 1 Balancing Nuclear Reactions Balance The Chegg Com




Chemical Composition Wt And Empiric Formula Apfu Si 4 Of Download Table




H Ch 4 Homework Key



File Periodic Table Zh Hk Svg Wikimedia Commons




The Role Of Atomic Oxygen In The Decomposition Of Self Assembled Monolayers During Area Selective Atomic Layer Deposition Sciencedirect




12 C 13 C Ratios And 29 Si 28 Si And 30 Si 28 Si Values Of Download Table




Science 09ruo Final Exam Review Using A Periodic Table Find The Atomic Mass And Write It Properly For Hydrogen 1 H Helium 4 He Carbon 12 C Oxygen Ppt Download




Home West Haven Board Of Education



Occurrence Of Quadruple Squamous Cell Carcinoma Following Allogeneic Hematopoietic Stem Cell Transplantation For Leukemia A Case Report
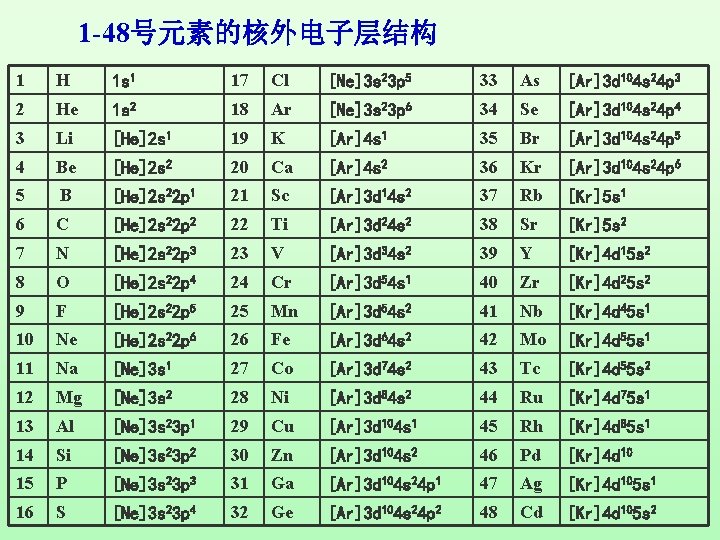



Chapter Seven Atomic Structure Atoms Neutrons Protons Positive



1
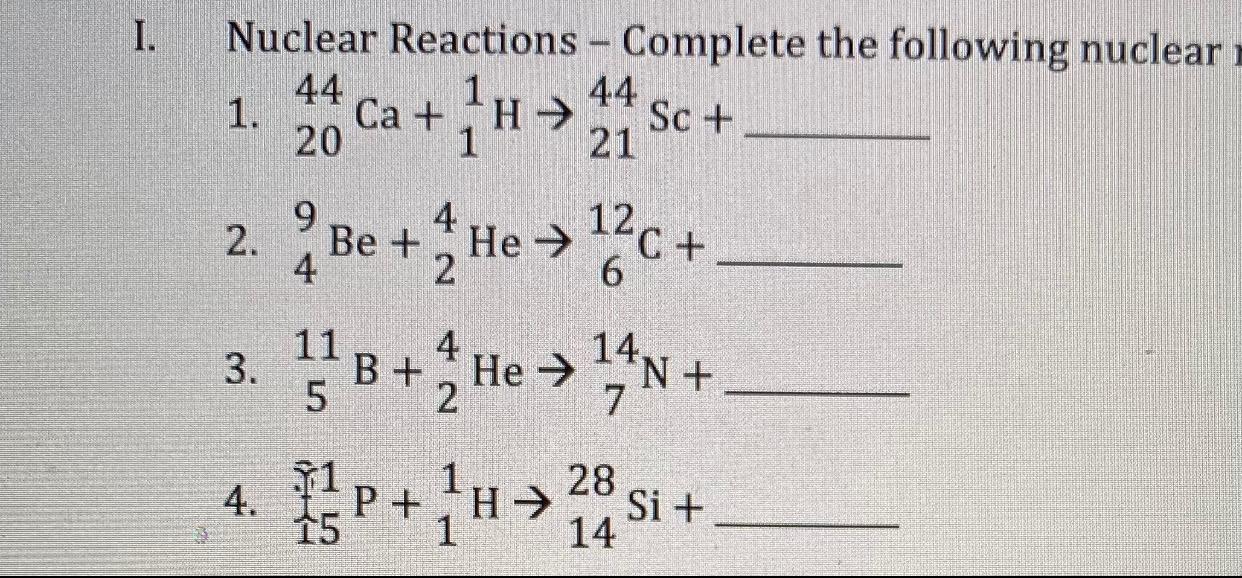



Solved I Nuclear Reactions Complete The Following Nuclear Chegg Com




Uc Worked Solutions Ch 02 Worked Solutions Chapter 2 Question 28 State The Number Of Protons Studocu



Plos One Photovoltaic Modules Evaluation And Dry Season Energy Yield Prediction Model For Nem In Malaysia




Bloomberg Markets 01 03 22 Youtube



Solved Esignating A N A Proton B Neutron Gamma Ray D Beta Particle E Alpha Particle 3 Which Of The Following Types Of Radiation Has The Highe Course Hero




Solved 16 Which One Of The Following Has The Biggest Atomic Radius A Fluorine B Caesium C Nitrogen D Lithium E Boron 17 The Condensed Electron Configuration Of Silicon Element 14 Is A He 2s42p6
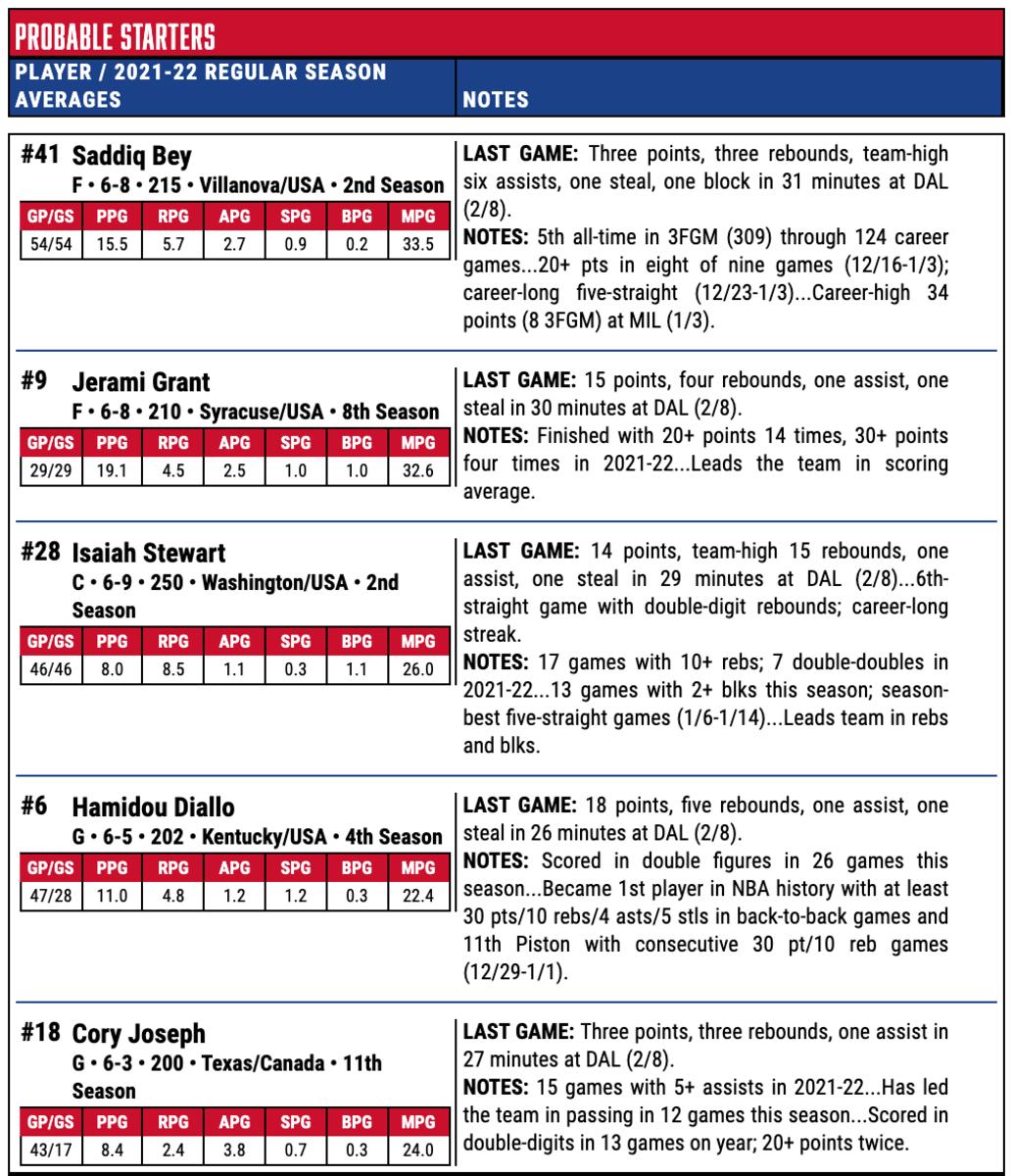



How To Watch Game Notes Quick Facts Injury Report Detroit Pistons Vs Memphis Grizzlies All Pistons



Plos One High Fat Diet Induced Insulin Resistance Does Not Increase Plasma Anandamide Levels Or Potentiate Anandamide Insulinotropic Effect In Isolated Canine Islets
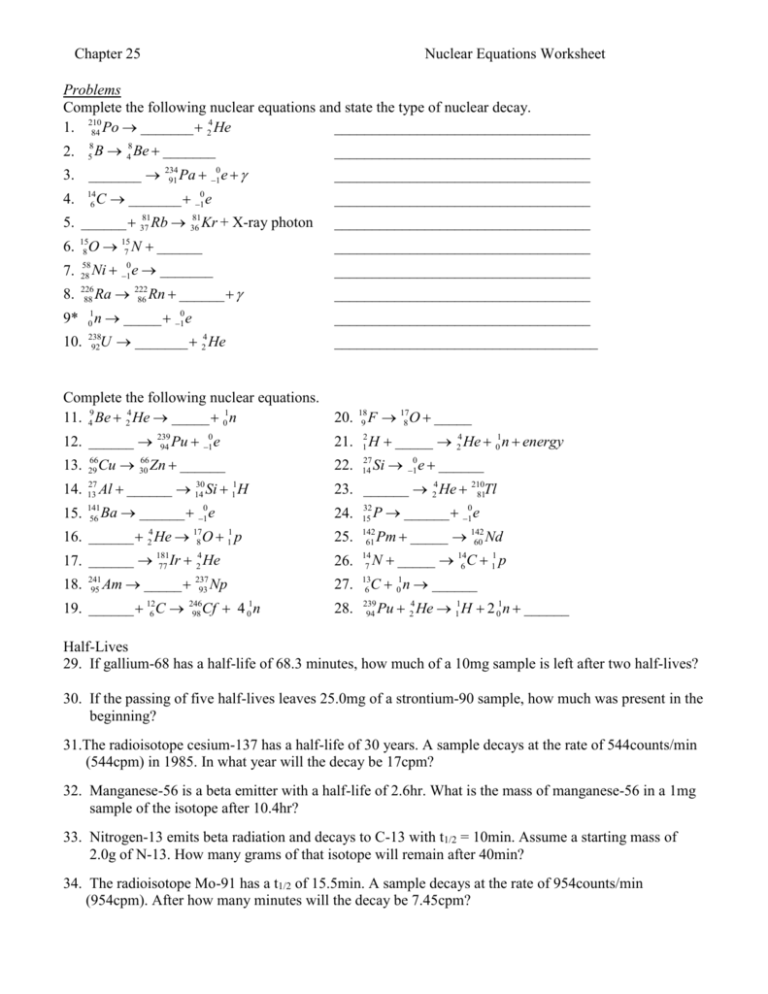



Nuclear Equations Worksheet




Solved 14 How Many Neutrons Are Present In Neon A 29 B Chegg Com



Bio Europe 22 Silicon Maps




In The Following Nuclear Reactions 7 N 14 2 He 4 Rarr 8 O 17 X 1 And 13 Al 27 1 D




Solved 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 1a 2a 3a Chegg Com
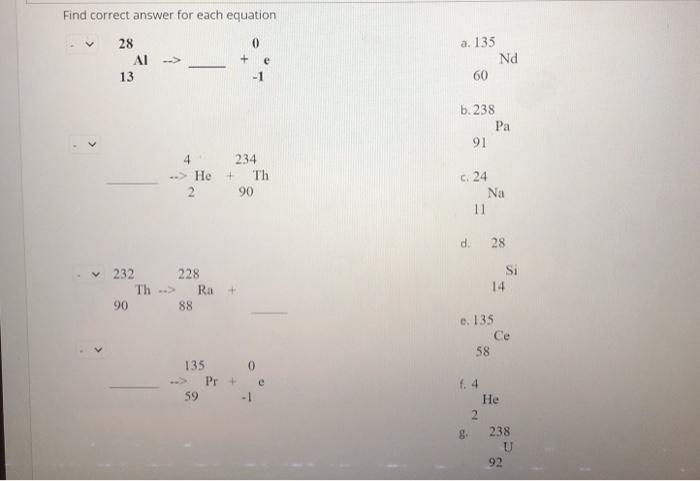



Solved Find Correct Answer For Each Equation 28 0 Ai 13 1 Chegg Com
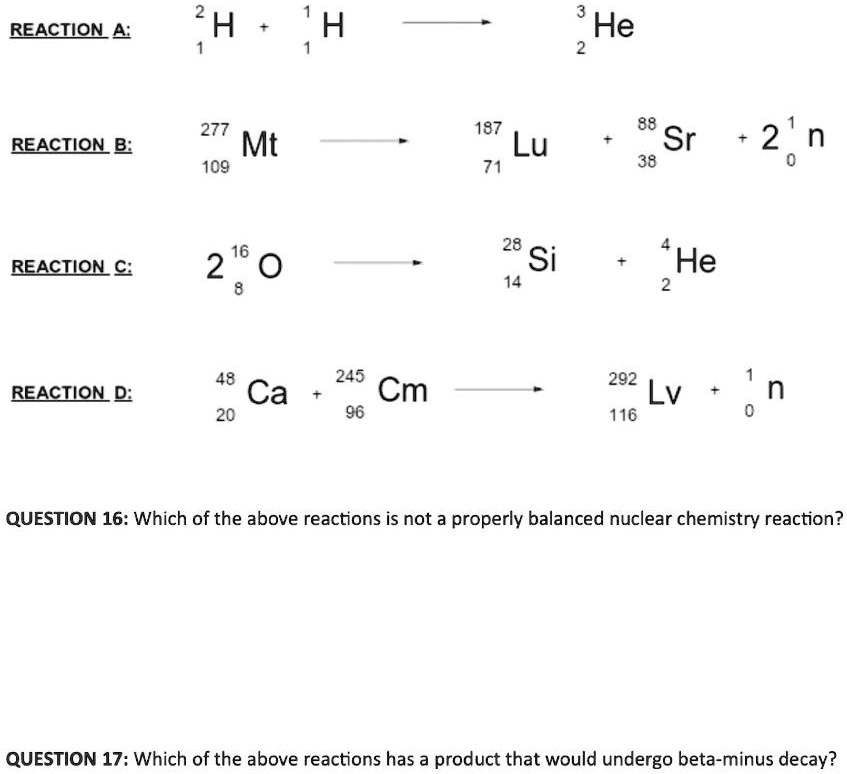



Solved Reaciion 4 H H He 277 Mt 109 187 Lu 71 Sr 38 Reaciion B 2 N 16 2 0 28 Si 14 Reaciion C He 48 Ca 245
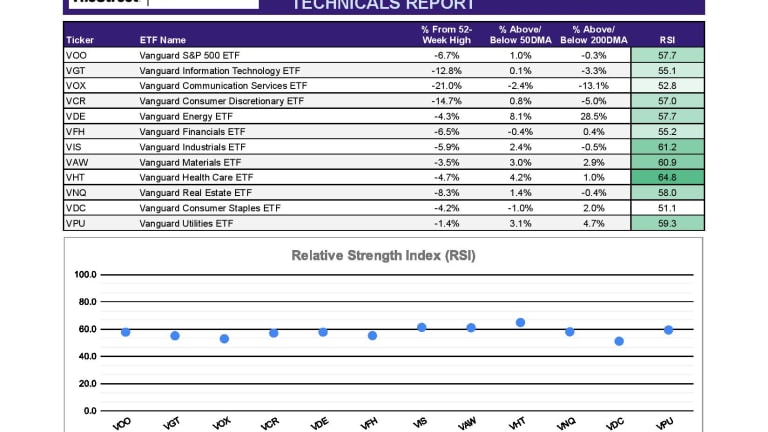



Don T Buy Into This Equity Rally Etf Focus On Thestreet Etf Research And Trade Ideas
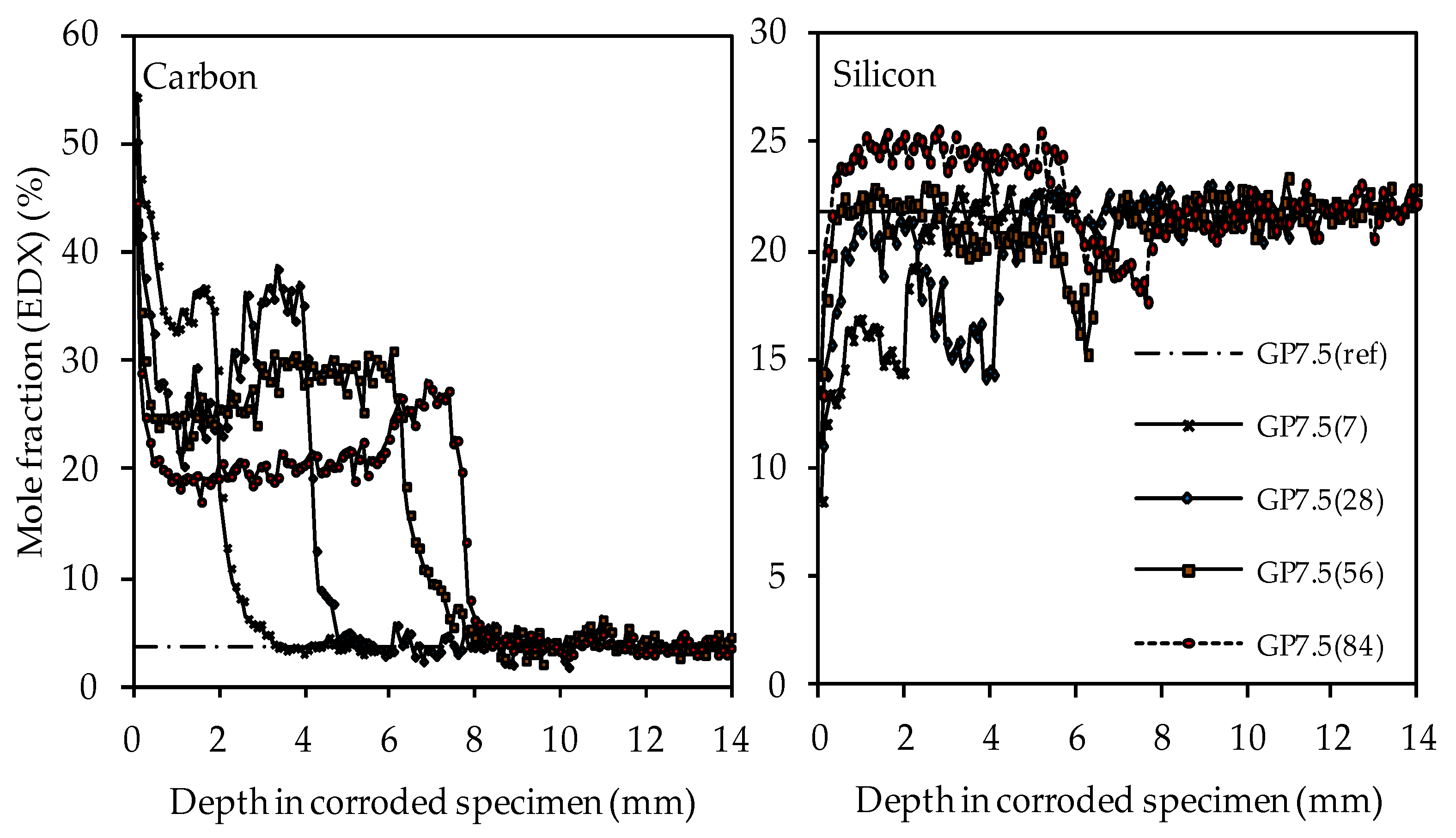



Materials Free Full Text Effect Of Silica Fume On Metakaolin Geopolymers Sulfuric Acid Resistance Html




Tanner Groves Powers Oklahoma Past Indiana State Sports Illustrated Oklahoma Sooners News Analysis And More




Book Of The Royal Blue 5 6 7 1 9 3 4 1 2 3 4 1 Lo 11 1 13 14 B 6 7 R 9 1 1 11 6
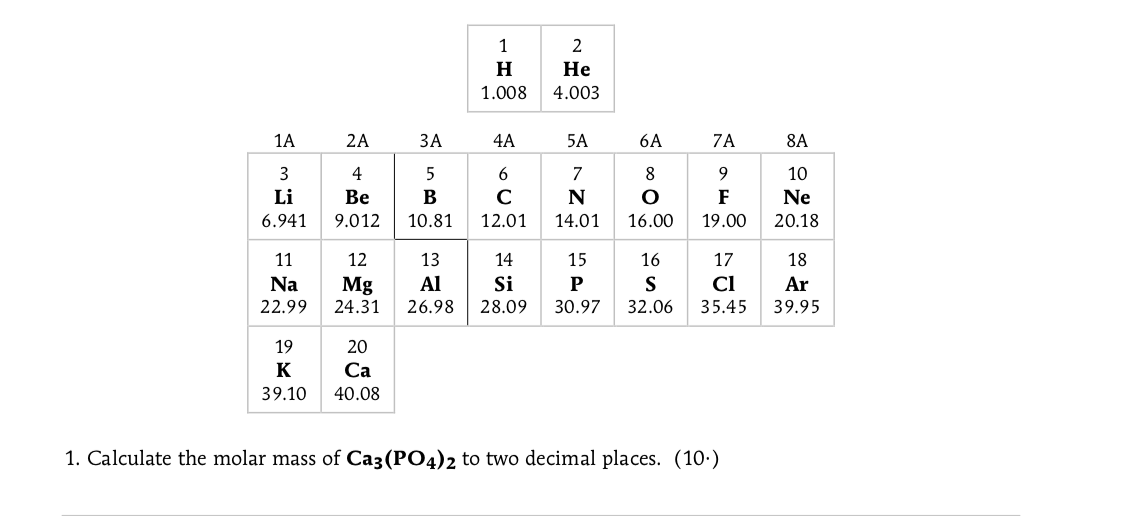



Solved 1 H 1 008 2 He 4 003 1a 2a Za 4a 5a 6a 7a 8a W Li Chegg Com
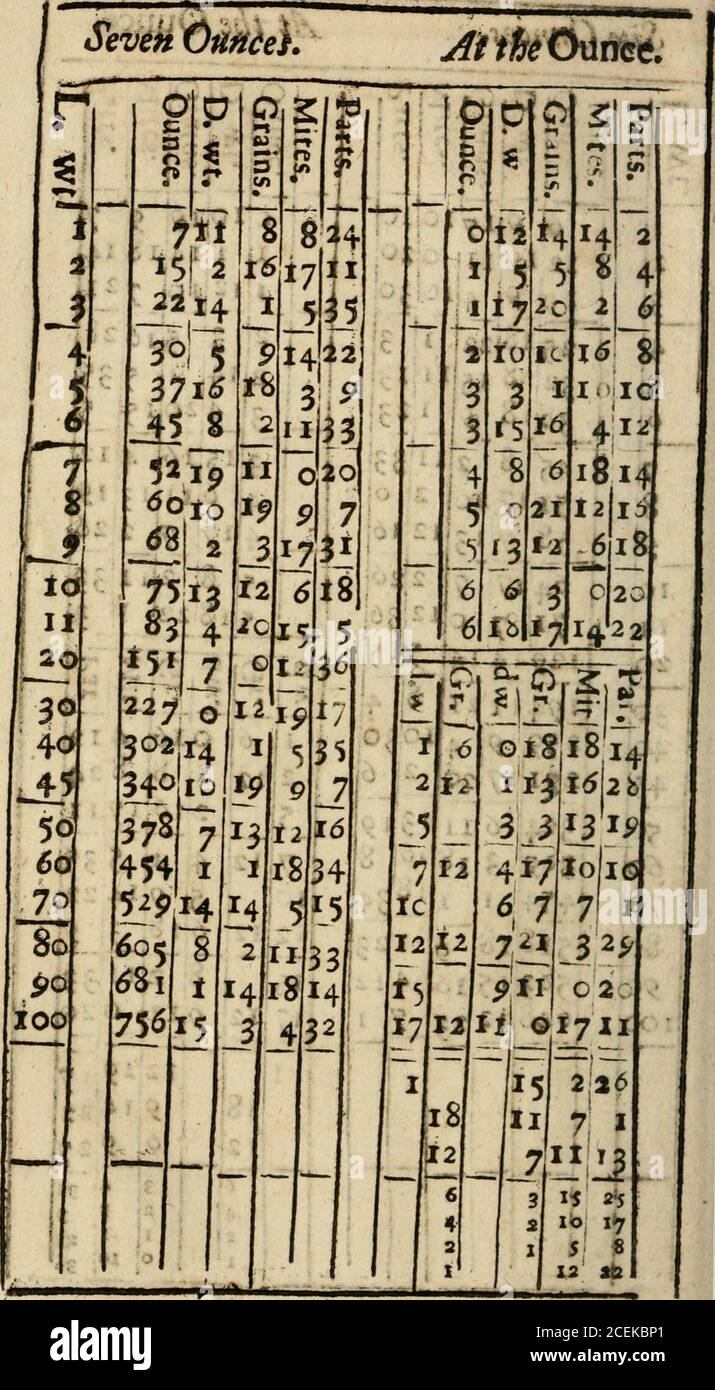



A New Touch Stone For Gold And Silver Wares 12 4 5 12 5 22 526 Pc 4b 17 10 10 15 1 18 3 9 100 9 17i
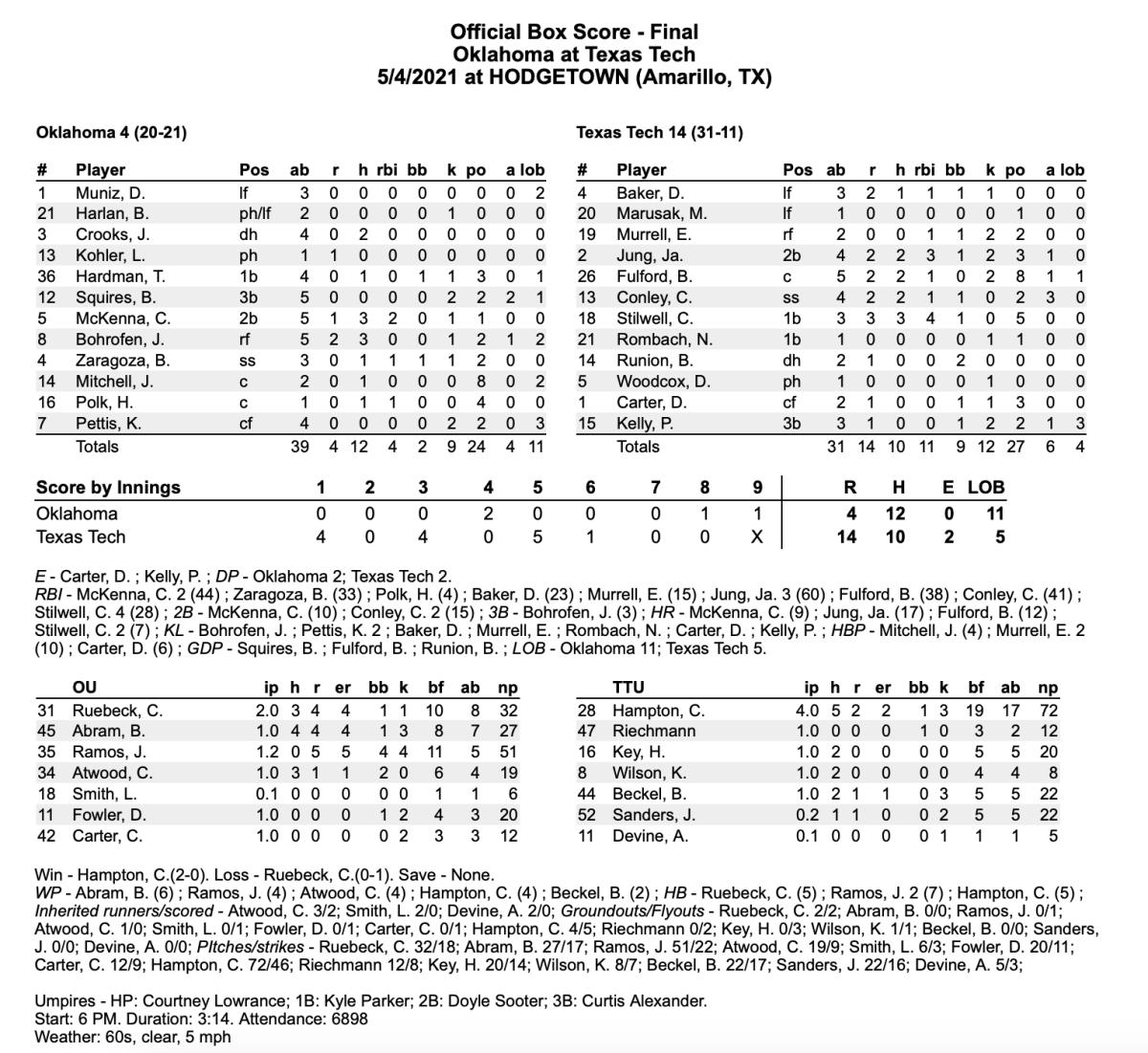



Oklahoma Sooners Routed By Texas Tech Red Raiders In Amarillo Sports Illustrated Oklahoma Sooners News Analysis And More
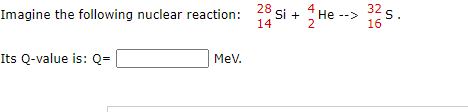



Solved Imagine The Following Nuclear Reaction 28 Si 14 4 Chegg Com




H30kpnoufdiztm




Silicon Podcast Chemistry World



0 件のコメント:
コメントを投稿